Introduction: Aggressive T-cell lymphomas (TCL) account for 10-15% of non-Hodgkin's lymphomas and have lower rates of complete response (CR), overall response (OR), and shorter duration of response (DOR) to chemotherapy than other types of NHL, particularly in the relapsed setting. Cutaneous T-Cell lymphoma (CTCL) is treated initially with skin directed therapies, but systemic therapy or cytotoxic chemotherapy may become necessary to control disease. Second-line chemotherapy benefits only a small minority of TCL patients and more effective treatment regimens are needed. Gemcitabine and liposomal doxorubicin have both shown single agent activity in TCL and have been used in combination in patients with relapsed B-cell lymphomas (BCL) and Hodgkin lymphomas (HL), though responses are typically not durable. Therefore, we evaluated a combination of gemcitabine and liposomal doxorubicin (GemDox) for patients with relapsed/refractory (r/r) TCL.
Methods: In this single-center, retrospective study, we evaluated the safety, efficacy, and survival (PFS/OS using Kaplan-Meier method) of GemDox in TCL. Patients either received gemcitabine 1000 mg/m 2 and liposomal doxorubicin 15 mg/m 2 either on days 1 and 8 every 21 days (21-day regimen) or gemcitabine 1000 mg/m 2 and liposomal doxorubicin 15 mg/m 2 on days 1 and 15 every 28 days (28-day regimen).
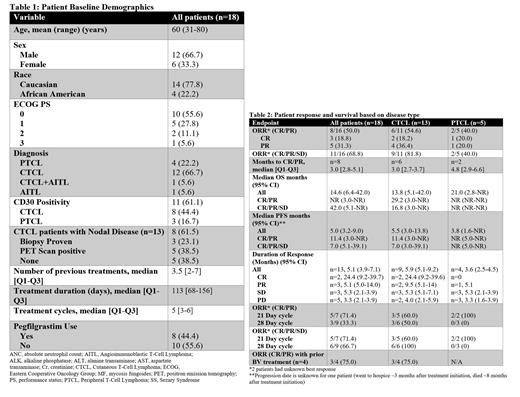
Results: 18 patients (13 CTCL, 5 PTCL) with r/r aggressive TCL were treated with GemDox, with 16 evaluable for treatment response. Patients were heavily pre-treated with a median number of 4 prior therapies (range 2-7). This included 62% of CTCL patients with nodal involvement. The overall response rate (ORR) was 50% (19% CR and 31% PR) with a median PFS and OS of 5 and 15 months respectively. In patients that had a response to treatment, the median PFS was 11 months while median OS was not reached. The clinical benefit rate [CR/Partial response (PR)/stable disease (SD)] for CTCL and PTCL was 82% and 40% respectively. The median PFS and OS for CTCL patients was 6 months and 14 months respectively, with an ORR of 55% (18% CR and 36% PR). For PTCL , the median PFS and OS were 4 and 21 months respectively, with an ORR of 40% (20% CR and 20% PR). The clinical benefit (CR/PR/SD) rate for the 21-day vs 28-day regimen was 71% and 67%, respectively, with similar PFS and OS, and no significant difference in toxicities. The median duration of response (DOR) for patients that obtained a response (CR/PR) in evaluable patients was 13 months; 17 months for CTCL, and 5 months for PTCL. We observed a substantial decrease in CTCL skin manifestations, as patients that had a CR/PR had a mean decrease of 76% in mSWAT BSA of 70%. Further, six patients (38%) (5 CTCL, 1 PTCL) were able to proceed to allogeneic stem cell transplant (allo-HSCT). Interestingly, 4 CD30+ CTCL patients were previously treated with brentuximab vedotin (BV). Of those, three (75%) had a response to GemDox, while only one achieved a response (PR) to BV. This demonstrates that GemDox is a potentially effective regimen in heavily refractory CD30+ CTCL patients that fail treatment with BV. 11 patients (61%) experienced any grade 3/4 toxicity and hematologic toxicities were the most common including: anemia (33%), neutropenia (22%), lymphopenia (17%) and thrombocytopenia (17%)., GemDox was very well tolerated with the most common grade 3/4 non-hematologic toxicity being fatigue (17%). 11% of patients discontinued therapy due to adverse events.
Conclusion: GemDox produced an excellent ORR of 50%, with an impressive ORR of 82% in CTCL, suggesting this is an effective regimen for heavily pre-treated TCL patients. Additionally, a large number of patients with CTCL with heavy skin burden, including those refractory to BV, were able to achieve remarkable skin responses. Importantly, in this study, 75% of patients who responded to GemDox were able to proceed to allo-HSCT, with 66% experiencing a sustained response. Based on these findings, GemDox should be further investigated as a therapeutic option in relapsed aggressive TCL, particularly in CTCL and considered as a potential bridging therapy patients with a treatment goal of obtaining allo-HSCT and providing a long-term benefit.
OffLabel Disclosure:
Porcu:Kyowa: Consultancy; Kymera: Membership on an entity's Board of Directors or advisory committees; BioGene: Membership on an entity's Board of Directors or advisory committees; Dren-Bio, ADCT, Lilly-Loxo, Viracta, Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Ono: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma: Consultancy; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma, Ono: Honoraria; Teva: Research Funding; Innate Pharma: Research Funding. Brammer:Verastem: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kymera: Consultancy; Incyte: Research Funding; Dren Bio: Consultancy; Bristol Myers Squibb: Research Funding.
Both gemcitabine and liposomal doxorubicin are chemotherapeutic agents that are recommended for individual use per NCCN guidelines, with proven efficacy but combined use is new and off-label.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal